A thorough understanding and strict adherence to the FDA requirements for whitening serums has become an absolute necessity for survival for skincare brands targeting the U.S. market. The U.S. Food and Drug Administration (FDA) has continuously tightened regulations, effectively moving from restrictions to a ban on hydroquinone in over-the-counter (OTC) products. Any misjudgment can lead to product seizure, warning letters, or legal action. This article provides an in-depth analysis of the framework for FDA requirements for whitening serums in 2026, offering brands a clear compliance roadmap.
I. Regulatory Foundation: The Core FDA Position That Must Be Understood First

Before delving into details, brands must be clear about the fundamental premise: the FDA requirements for whitening serums are built on the principle of “safety first.” Currently, no OTC whitening serum is FDA-approved.
1.Core Ban: The Illegal Status of Hydroquinone in OTC Products
This is the most critical aspect of the FDA requirements for whitening serums. Under the CARES Act effective since 2020, all OTC skin-lightening products containing hydroquinone have been reclassified as “new drugs.” This means marketing such products is illegal unless a brand completes the lengthy and costly New Drug Application (NDA) process and gains approval. The FDA has issued warning letters to several non-compliant companies.
2.Absolute Red Line: Strict Prohibition of Mercury and Other Heavy Metals
The FDA requirements for whitening serums explicitly forbid adding any form of mercury (e.g., mercurous chloride, calomel). Mercury is highly toxic to the human nervous system, kidneys, and more. Consumers should watch for aliases like “mercurous chloride” or “calomel” on ingredient lists.
3.The Sole Exception: The Prescription Drug Pathway
Currently, the only FDA-approved product containing hydroquinone in the U.S. is the prescription drug Tri-Luma, approved only for the short-term treatment of moderate to severe melasma-related dark spots.
II. In-Depth Analysis: The Safety Logic Behind Stringent FDA Requirements
The strict nature of the FDA requirements for whitening serums stems from the assessment of proven risks and the mandate to protect consumer health.
1.Confirmed Health Hazards from Non-Compliant Products
FDA-collected adverse event reports indicate that using OTC products containing hydroquinone can lead to:
- Exogenous Ochronosis: A potentially permanent blue-black skin pigmentation.
- Severe skin irritation, rashes, and contact dermatitis.
- Abnormally increased skin sensitivity.
2.Long-Standing Carcinogenicity Concerns
As early as 2006, the FDA raised concerns about hydroquinone’s potential carcinogenic risk based on animal study data. Although human evidence remains inconclusive, this concern forms the historical context for its cautious regulation.
3.Control Over Unsupervised Misuse
Consumers lacking professional guidance may use products excessively over long periods, amplifying risks. The FDA requirements for whitening serums aim to confine such potent ingredients strictly to medically supervised settings.
III. Brand Action Guide: Developing Compliant Whitening Serums Within the FDA Framework
Faced with clear FDA requirements for whitening serums, brands must adjust R&D, claims, and market entry strategies.
1.Formulation Strategy: Shift to Safe and Effective Alternative Ingredient Systems
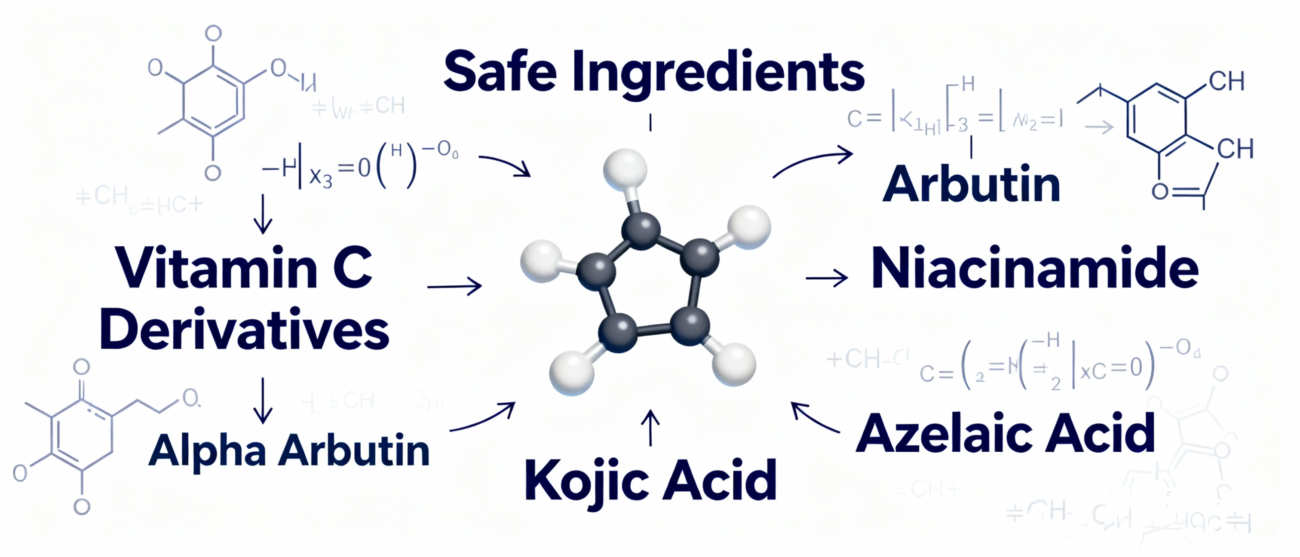
Abandon hydroquinone in OTC products entirely. Focus should shift to ingredients with well-established safety records and clear mechanisms of action:
- Antioxidation & Inhibition of Melanin Production: Use stable Vitamin C derivatives, kojic acid, arbutin, etc.
- Blocking Melanin Transfer: Niacinamide is a core, research-validated safe choice.
- Accelerating Keratinocyte Renewal: Use alpha-hydroxy acids or salicylic acid (mind concentration limits) appropriately to help fade surface pigment.
2.Claim Strategy: Precise Wording, Strictly Adhering to “Cosmetic” Boundaries
Product claims must strictly comply with the cosmetic definition. Any statement implying the product can “treat” or “cure” a skin disease will cause it to be classified as a drug.
- Examples of Compliant Claims: “Helps brighten skin tone,” “improves the appearance of dullness,” “helps reduce the appearance of sun-induced dark spots.”
- High-Risk Claims to Avoid: “Treats melasma,” “removes freckles,” “blocks melanoma,” “provides clinical-grade whitening effects.”
3.Compliance Pathway Options: The Only Two Roads
According to the FDA requirements for whitening serums, brands have only two paths:
- The Mainstream Path: Develop Compliant OTC Cosmetics
- The Niche Path: Pursue a New Drug Application
IV. Market Adaptation and Consumer Communication: Turning Compliance into a Trust Asset
In the new regulatory environment, leading brands should proactively internalize the FDA requirements for whitening serums as a brand responsibility.
1.Proactive Education to Build a Transparent Image
Explain to consumers why safer alternatives are chosen, educate them on the risks of hydroquinone and mercury, and transform compliance into a reputation for professionalism and responsibility.
2.Guiding Consumers to Identify Non-Compliant Products
Help consumers develop discrimination skills to be wary of products with incomplete labeling, overly aggressive claims, or from unknown sources.
3.Advocating for a Healthy Skincare Philosophy
Shift the market focus from “skin whitening” alone to “skin tone evenness, skin health, and overall radiance,” aligning with the FDA’s mission to protect public health.
V. FAQ: Deepening Understanding of FDA Requirements
Are there currently any FDA-approved OTC whitening serums?
No, currently there are none. The FDA requirements for whitening serums clearly state that all products marketed as OTC whitening serums containing hydroquinone are illegally sold.
Is a whitening serum free of hydroquinone and mercury fully compliant with FDA requirements?
Avoiding these two prohibited ingredients is the foundation of compliance, but it’s not the entirety. Brands must also ensure all ingredients are safe at claimed concentrations and all claims are strictly within the cosmetic category.
How should a brand respond if it receives an FDA Warning Letter concerning a whitening product?
Typically, the FDA requires a company to respond within 15 working days with a plan for immediate cessation of sales, market removal, and corrective measures.
How might the FDA requirements for whitening serums evolve in the future?
The FDA is expected to continue strengthening enforcement against illegal OTC products, especially those sold online or imported, with higher demands for safety and scientific substantiation.
VI. Conclusion and Outlook: Finding Innovation Opportunities Within Compliance
In 2026, the FDA requirements for whitening serums draw clear boundaries for the industry: safety is the absolute red line, and compliance is the only entry point.
- Uphold the Compliance Baseline: Completely abandon any use of hydroquinone or mercury in OTC products.
- Focus on Scientific Innovation: Increase investment in research on safe whitening actives, building competitiveness through solid scientific evidence.
- Build a Trust Asset: Make transparency, consumer education, and responsible claims central to brand communication.
Future successful whitening brands in the U.S. market will be those that not only understand the FDA requirements for whitening serums but also use them as a cornerstone to create genuine value within the framework of safety, science, and integrity.
If you are looking for a reliable skincare OEM manufacturer to empower your product compliance and success, we invite you to contact us.