As we enter 2026, European requirements for sunscreens continue to deepen and refine within the existing stringent regulatory framework. While the overall regulatory system of “European requirements for sunscreens” has not undergone a sudden overhaul in 2026, multiple bans and restrictions that came into force in previous years will enter their full implementation phase in 2026. At the same time, new scientific assessments are paving the way for future regulatory updates. For cosmetic brands, manufacturers, and exporters, accurately grasping these evolving “European requirements for sunscreens” is key to ensuring product compliance and smooth market access to the EU.
Quick Overview of European Requirements for Sunscreens
| Requirements Dimensions | Core Content | Key Timelines and Explanations |
| Banned Ingredients | 4-Methylbenzylidene Camphor | From May 1, 2026, all products containing this ingredient must be completely removed from the market. |
| Certain Benzophenones | Benzophenone-1 has been evaluated as unsafe. The safety of Benzophenone-2 is unconfirmed and poses a high risk. Use is not recommended. | |
| PFAS (Per- and Polyfluoroalkyl Substances) | From August 12, 2026, PFAS will be completely banned in cosmetic products, which may affect certain film-forming agents or water-resistant formulations. | |
| Restricted Ingredients | Homosalate | The maximum concentration in facial products (non-spray) must not exceed 7.34%. New products must comply from January 2025, and existing stock must be sold out by July 2025. |
| Labeling & Claims | UVA Protection Indication | It is recommended to use the standardized “UVA” circle logo to clearly inform consumers that the product complies with EU UVA protection standards (critical wavelength ≥ 370 nm, or PPD/SPF ≥ 1:3). |
| Prohibited Absolute Claims | The use of misleading terms such as “100% sun protection”, “complete block”, and “all-day protection” is strictly prohibited. | |
| Basic Regulations | Safety Assessment and Notification | A Product Safety Assessment (CPSR) must be completed, a Product Information File (PIF) established, and notification submitted via the Cosmetic Products Notification Portal (CPNP). |
1.Regulatory Framework and Compliance Basis
All sunscreens sold on the European market must first comply with the core framework of “European Requirements for Sunscreens”.
The central role of cosmetic regulations.
In the EU, sunscreens are explicitly classified as cosmetics and governed by the EU Cosmetics Regulation (EC) No 1223/2009. This means that the full responsibility for their safety lies with the responsible person, and products must meet a set of mandatory requirements before being placed on the market.
Mandatory Compliance Steps
The responsible person must ensure that each product has undergone a safety assessment by a qualified individual and that a detailed Product Information File (PIF) has been established for inspection. Furthermore, prior to placing a product on the market, a unified notification must be submitted via the EU Cosmetic Products Notification Portal (CPNP), which serves as the passport for the product to legally enter the EU market.
2.Ingredient Safety: Dynamically Updated Whitelists and Bans
The most dynamic aspect of the “European Requirements for Sunscreens” lies in the safety management of ingredients such as UV filters, which is the top priority for compliance work in 2026.
Explicit Bans Effective in 2026
- 4-MBC Phase-Out: Due to safety concerns such as endocrine-disrupting properties, the final sales deadline for products containing the UV filter 4-Methylbenzylidene Camphor (4-MBC) is May 1, 2026. Any product containing this ingredient on the market after this date will be deemed non-compliant.
- Ban on PFAS: As part of the control over persistent chemicals, the EU will ban the use of PFAS in cosmetics starting August 12, 2026. This requires formulators to review and remove such substances that may be used to enhance product water resistance or film-forming properties.
Risk Ingredients Under Strict Review
The EU Scientific Committee on Consumer Safety (SCCS) continuously re-evaluates multiple UV filters, and its conclusions directly impact regulations:
- Benzophenone Family: Benzophenone-1 has been definitively assessed as unsafe by the SCCS. Benzophenone-2 has not been confirmed safe due to observed genotoxicity and estrogenic activity, posing a very high risk. While Benzophenone-3 has specific restrictions in regions such as the UK, safety controversies persist at the EU level.
- Concentration Limit for Homosalate: The permitted concentration of this ingredient has been tightened. Current “EU Requirements for Sunscreens” stipulate that in facial care products (excluding sprays), its maximum concentration must not exceed 7.34%.
3.Strict Regulations on Efficacy Testing and Label Claims
The “EU Requirements for Sunscreens” not only focus on safety but also strictly regulate the efficacy verification and information communication of products, ensuring that consumers receive genuine and effective protection.
Standardized Efficacy Testing
The determination of sun protection factors must follow internationally recognized standard methods:
- The SPF value (UVB protection efficacy) is typically measured in accordance with standards such as ISO 24444 (in vivo testing) or the newly published ISO 23675 (in vitro testing).
- UVA protection efficacy can be demonstrated via in vitro methods like ISO 24443, proving either a Critical Wavelength (CW) ≥ 370 nm, or a ratio of the UVA Protection Factor (PPD) to the SPF value of at least 1:3.
Clear and Standardized Labeling Requirements
The European Commission Recommendation 2006/647/EC provides specific guidance on labeling. Although not a direct legal provision, it has become a prevailing market standard:
- Protection Level Identification: It is recommended that the SPF value be labeled together with protection category terms such as “low”, “medium”, “high”, and “very high”.
- UVA Logo: To indicate that the product offers EU-compliant UVA protection, the standardized UVA circle logo, promoted by the Cosmetics Europe association, is widely adopted.
- Mandatory Warnings and Instructions: Must include usage instructions such as “Keep infants and young children out of direct sunlight”, “Apply before exposure”, and “Reapply regularly”.
Misleading Claims Strictly Prohibited
Regulations explicitly ban any claims that may give consumers a false sense of absolute safety, such as:
- “100% sun protection” or “complete block”
- “All-day protection” or “lasts all day”
4.Other Important Regulations Related to Sunscreens
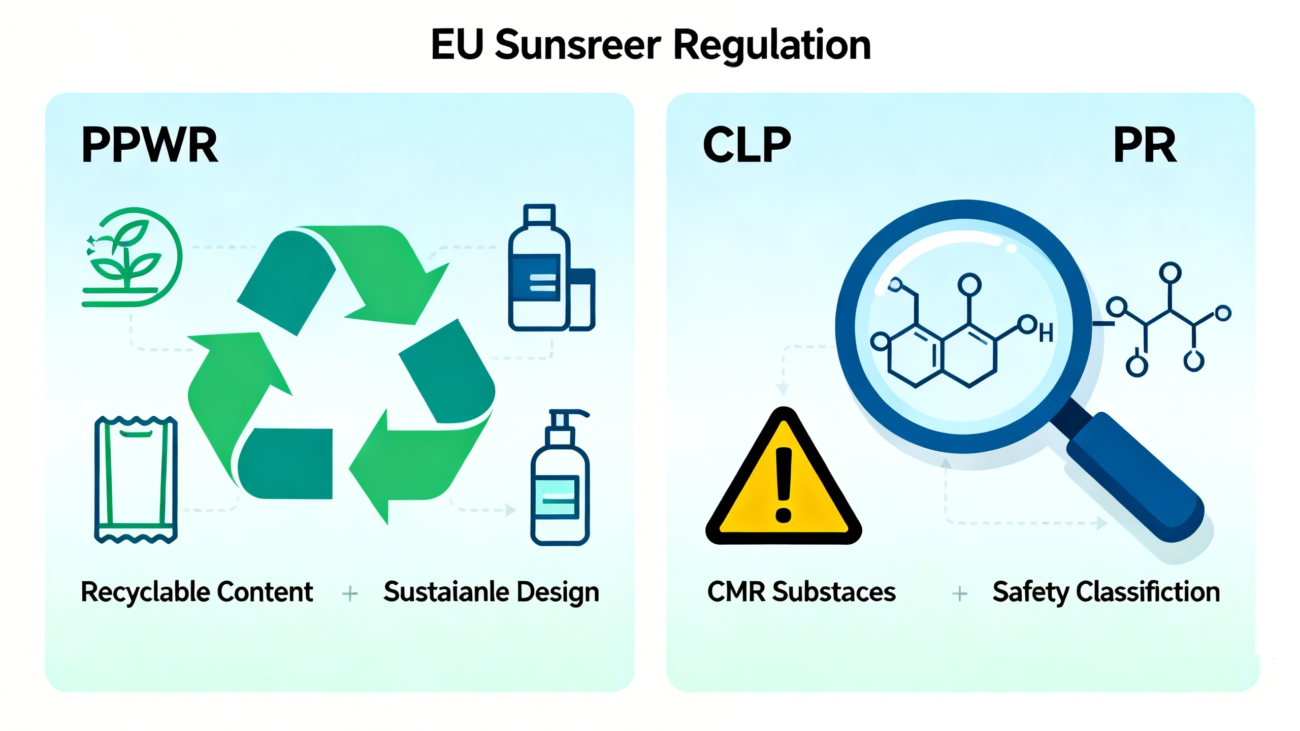
The “European Requirements for Sunscreens” are also reflected in broader horizontal regulations, which impact product packaging and ingredient selection.
Packaging and Packaging Waste Regulation (PPWR)
The EU’s Packaging and Packaging Waste Regulation (PPWR) sets ambitious recycling targets, such as requiring at least 70% recyclable content in all packaging by 2030. This requires brands to consider environmental requirements from the very beginning of packaging design.
Classification, Labelling and Packaging Regulation (CLP)
Updates to the CLP Regulation will classify more substances as carcinogenic, mutagenic, or toxic for reproduction (CMR). Once listed, these substances are generally banned from use in cosmetics. Companies must continuously monitor updates to the relevant lists.
Summary
In summary, the “European Requirements for Sunscreens” for 2026 are characterized by a stable regulatory framework with increasingly stringent standards. The main challenges for businesses stem from the continuously rising safety thresholds for ingredients (such as the final phase-out of 4-MBC, the ban on PFAS, and the strict review of benzophenones), as well as growing standards in sustainability and information transparency.
For relevant companies, a proactive compliance strategy is crucial: immediately review and reformulate products to remove or replace high-risk ingredients; ensure rigorous compliance with testing methods and label claims; and continuously monitor scientific opinions from the SCCS and official EU announcements to adapt to potential future regulatory updates. Only by thoroughly understanding and proactively meeting these “European Requirements for Sunscreens” can products establish a solid foothold in the EU market.
If you have sunscreen OEM needs, please feel free to contact us, and let us empower your products.