The EU Requirements for Skincare Products are renowned globally for their universally recognized strictness, systematicity and forward-looking nature. For any skincare brand planning to enter or maintain operations in the European market, gaining a thorough understanding of and strictly complying with these requirements constitutes not only a legally mandatory obligation, but also a cornerstone for establishing product safety credibility and earning consumer trust. In 2026, the EU regulatory framework will continue to evolve toward digitalization, transparency and sustainability, building on its existing core regulations. This article provides a comprehensive analysis of the current and upcoming key provisions of the EU Requirements for Skincare Products, offering clear guidance for your compliance journey.
Ⅰ.Core Framework of the EU Regulatory System for Skincare Products
The European Union’s regulation of cosmetics is built on a unified regulatory framework, ensuring consistent standards across its 27 member states. This grants businesses the convenience of one-time compliance, cross-Union market access, while also setting an extremely high compliance threshold.
1.Core Regulation: EU Cosmetic Regulation (EC) No 1223/2009
This regulation serves as the cornerstone of the regulatory regime, covering the entire product lifecycle from product safety assessment, ingredient use, good manufacturing practice, labeling and marking, to market surveillance. Its core principle is to ensure that cosmetics placed on the market are safe for human health under normal or reasonably foreseeable conditions of use.
2.Key Supporting Systems and Databases
- Cosmetic Ingredient Database (CosIng): This is the primary official tool for checking restrictions on ingredient use, ingredient functions, and whether substances are banned or restricted. It serves as an essential reference for enterprises when developing formulations.
- Cosmetic Products Notification Portal (CPNP): All cosmetics marketed in the European Union must complete an online notification via this system prior to market placement. This is a mandatory administrative procedure.
- Classification, Labelling and Packaging Regulation (CLP Regulation): It applies to hazardous mixtures, such as certain high-strength exfoliating products. It mandates the classification, labelling of such products and the completion of safety data sheets in accordance with uniform rules.
II. Detailed Explanation of Core Compliance Requirements
To legally market skincare products in the European Union, the following specific and interrelated EU Requirements for Skincare Products must be satisfied.
1.Product Safety and Responsible Person
- Mandatory Appointment of a Responsible Person: Every cosmetic product placed on the EU market must have a Responsible Person established within the European Union. This individual or entity is responsible for ensuring and demonstrating that the product complies with all regulatory requirements, and serves as the primary point of contact for regulatory authorities.
- Product Safety Report: This is the technical core. The Responsible Person must hold a Product Safety Report (PSR) for each product, which consists of:
- Part A: Product Safety Information:Complete product formulation, chemical and toxicological properties of raw materials, product stability testing, microbiological quality, packaging material information, normal and reasonably foreseeable conditions of use, exposure assessment, adverse event history, among other items.
- Part B: Product Safety Assessment:A qualified safety assessor shall issue a professional assessment conclusion on the safety of the product for human health, based on the information set out in Part A.
2.Ingredient Management and Restrictions
The European Union implements a strict regulatory model for cosmetic ingredients that combines a negative list and positive list control.
- List of Banned Substances: More than 1,600 substances are explicitly prohibited from use.
- List of Restricted Substances: Strict regulations are imposed on the permitted concentration, conditions of use (e.g., for rinse-off products only) and required warning statements, with examples including salicylic acid and certain preservatives.
- Lists of Permitted Colorants, Preservatives and UV Filters: Only substances included in the corresponding lists may be used for their designated functions.
3.Product Information File and CPNP Notification
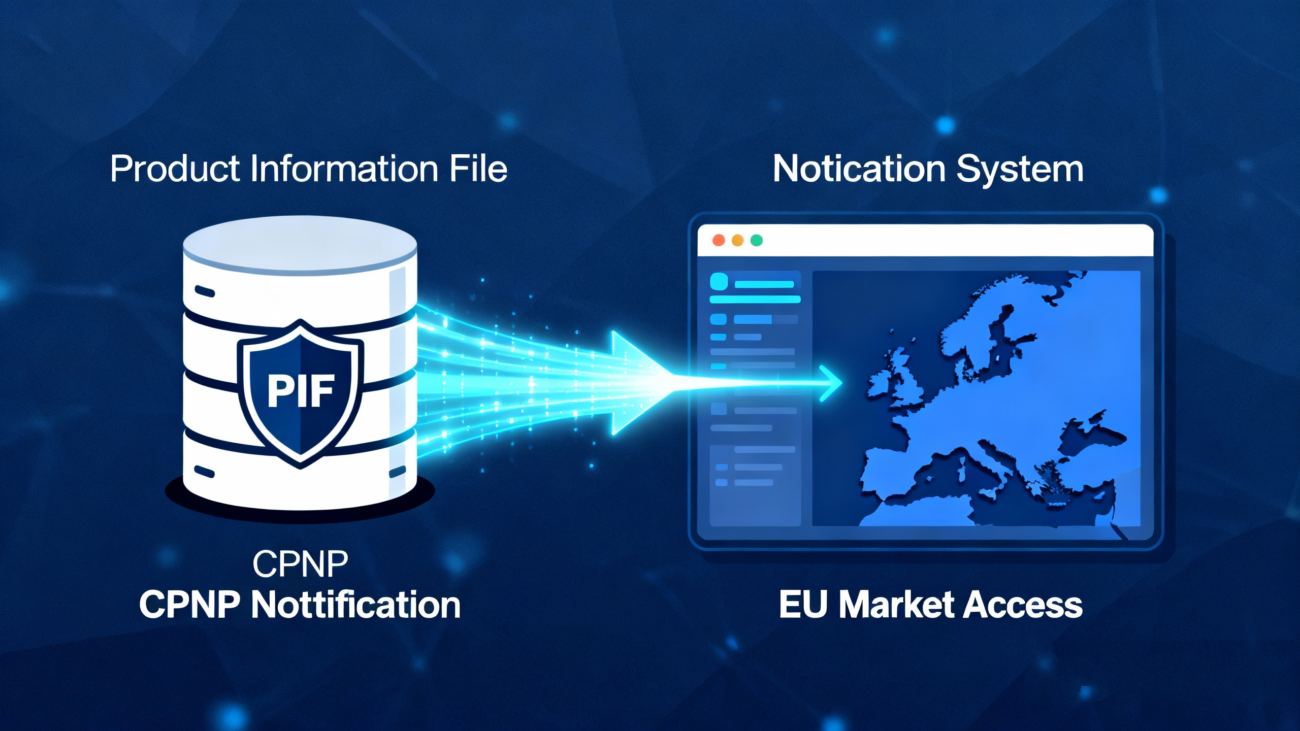
Compliance as an “evidence package” and “identity registration”.
- Product Information File: It must contain all evidence and data supporting the Product Safety Report, and shall be retained for at least 10 years starting from the date the last batch of the product is placed on the market, for immediate inspection by the competent national authorities at any time.
- CPNP Notification: Prior to placing the product on the market, the Responsible Person shall submit information including product category, product name, information on the presence of nanomaterials, label photographs and other details via this system. The product may be placed on the EU market upon completion of the notification, with no need to wait for official approval.
4.Labelling and Product Claims
This serves as the primary channel for consumers to obtain product information, with extremely detailed regulatory requirements.
- Mandatory Labelling Information: The information must be printed in the official language(s) of the EU member state where the product is sold. It shall be clearly visible, prominent and indelible, including: the name and address of the Responsible Person, country of origin, quantity, shelf life or period after opening (PAO), precautions, batch number, ingredient list, among other items.
- Ingredient List (INCI): All ingredients shall be listed in descending order of concentration. Ingredients with a concentration of 1% or less may be listed in any order. Colorants may be listed at the end.
- Compliance of Product Claims: All efficacy claims made for products must be lawful, truthful and substantiated. They must not imply that the product possesses medicinal properties. The European Union has issued the Common Criteria for Cosmetic Claims, which mandate that claims must be supported by publicly accessible evidence.
III. Key Focus Areas and Emerging Trends in 2026
In addition to maintaining static compliance, enterprises must also dynamically monitor the development of EU policies.
1.Full Implementation and Challenges of the Animal Testing Ban
The European Union has implemented a complete ban on animal testing for finished cosmetic products and their ingredients. This stance remains firm in 2026. This means that enterprises must rely on in vitro methods, computer models, or existing human safety data to demonstrate product safety, imposing extremely high compliance requirements on ingredient suppliers in the upstream supply chain.
2.Deepening Sustainability and Green Transition
The European Union is promoting a circular economy through a series of policies, and the relevant EU Requirements for Skincare Products are becoming increasingly stringent.
- Packaging and Waste Regulations: Affected by regulations including the Packaging and Packaging Waste Regulation, brands are required to prioritize packaging reduction, adopt recyclable materials, and fulfill extended producer responsibility.
- Environmental Footprint and “Green Claims”: “Green claims” related to environmental impacts, such as product carbon footprint and biodegradability, will be subject to stricter scrutiny to prevent greenwashing.
3.Exploration of Digital Labelling
To deliver more comprehensive product information that can be updated in multiple languages while reducing packaging printing waste, the European Union is actively exploring the potential of digital labelling. While it may not become a mandatory requirement in 2026, early understanding and preparation are highly forward-looking.
IV. Practical Recommendations for Enterprises
To tackle the complex EU Requirements for Skincare Products, a systematic response is critical.
- Appoint an EU Responsible Person at the earliest opportunity: This is a prerequisite for launching all compliance-related work.
- “Compliance starts with R&D”: During the product formulation development stage, utilize tools such as the CosIng database to screen ingredients and avoid the use of controversial or restricted substances.
- Collaborate with experienced partners: For non-EU enterprises, partnering with third-party compliance service providers, lawyers or seasoned importers that are well-versed in EU regulations is an efficient and risk-mitigating option.
- Establish and maintain dynamic compliance files: Develop a complete compliance file for each product, and update it continuously as regulations are revised, formulations are fine-tuned, or new safety data becomes available.
- Monitor industry developments and official updates: Regularly consult the official website of the Directorate-General for Health and Food Safety of the European Commission and authoritative industry information sources to ensure information remains up-to-date.
Ⅴ.Conclusion
The EU market continues to attract global brands thanks to its high consumer purchasing power and well-regulated business environment. However, its stringent regulatory framework sets a high barrier to market entry. Gaining an in-depth understanding and systematically meeting the EU Requirements for Skincare Products is far more than just passing inspections; it has become a core component of product competitiveness and brand reputation. In 2026, only by deeply integrating compliance into product development and brand strategy can enterprises achieve sustainable growth in the European market, turning regulatory challenges into solid competitive advantages.
If you intend to build your own skincare brand, DESIFINE can provide you with professional support. Please feel free to contact us to share your plans.